Good resource allocation
I. Inventory and dispatch of epidemic prevention supplies:
(I) Compiled a list of products with product licenses for medical devices necessary in the fight against the disease, and actively investigated domestic demand and sales.
(II) Closely monitored the daily domestic medical alcohol production and distribution, and coordinated with Taiwan Tobacco & Liquor Corporation and Taiwan Sugar Corporation to produce alcohol for general consumers to use for cleaning needs, as well as to meet the demand for medical and daily use.
II. Face masks are requisitioned and reasonably distributed by the government:
(I) Face masks for private use:
- Name-Based Mask Distribution System 1.0 was implemented on February 6, 2020. The system allowed citizens to bring their NHI card to purchase face masks at over 6,000 NHI-contracted pharmacies and over 300 health centers. To make resource allocation fairer, the National Health Insurance Administration (NHIA) built the mask management system under the NHI VPN system for sales distribution, and the Central Epidemic Command Center (CECC) adjusts the distribution of masks every week based on actual demand. Furthermore, the NHIA also releases updates on the mask stocks at every NHI-contracted pharmacy on the Government Open Data Platform. This allows the private industry to develop various value-added applications, so the public can easily find out the location of pharmacies and their stocks of mask.
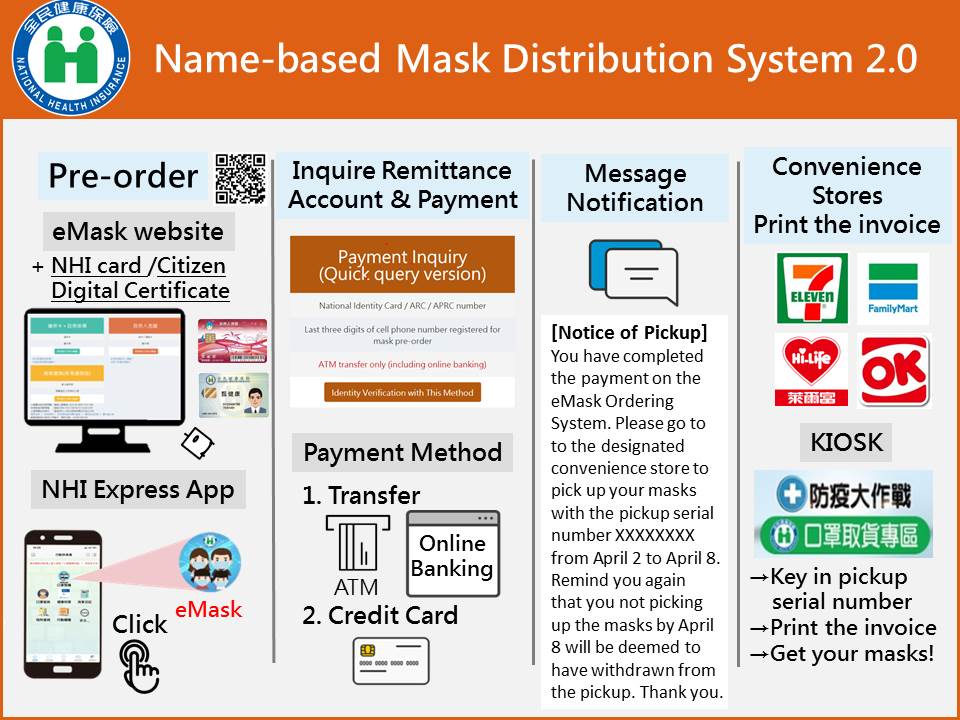
- Name-Based mask Distribution System 2.0 was implemented on March 12, 2020 (ceased on October 24, 2021). Citizens can order masks after completing the identity verification through the NHI App on the mobile phone, or logging in to the “eMask Ordering System” with their NHI card or Citizen Digital Certificate on the computer.
- Name-Based mask Distribution System 3.0 was implemented on April 22, 2020 (ceased on October 24, 2021). Citizens can order masks at the kiosk using their NHI card at over 10,000 convenience stores around Taiwan, which reduced the waiting time, and made the process of purchasing masks more convenient.
- As of Apr. 30, 2022, in the “Name-Based Mask Distribution System 1.0", a total of about 1,432 million masks were sold. As of Oct. 11, 2021, in the “Name-Based Mask Distribution System 2.0 and 3.0", about 6.2 million people have ordered masks through the Internet or convenience stores, and a total of about 231.52 million masks were sold.
(Name-based Mask Distribution System 1.0)
(Name-based Mask Distribution System 2.0)
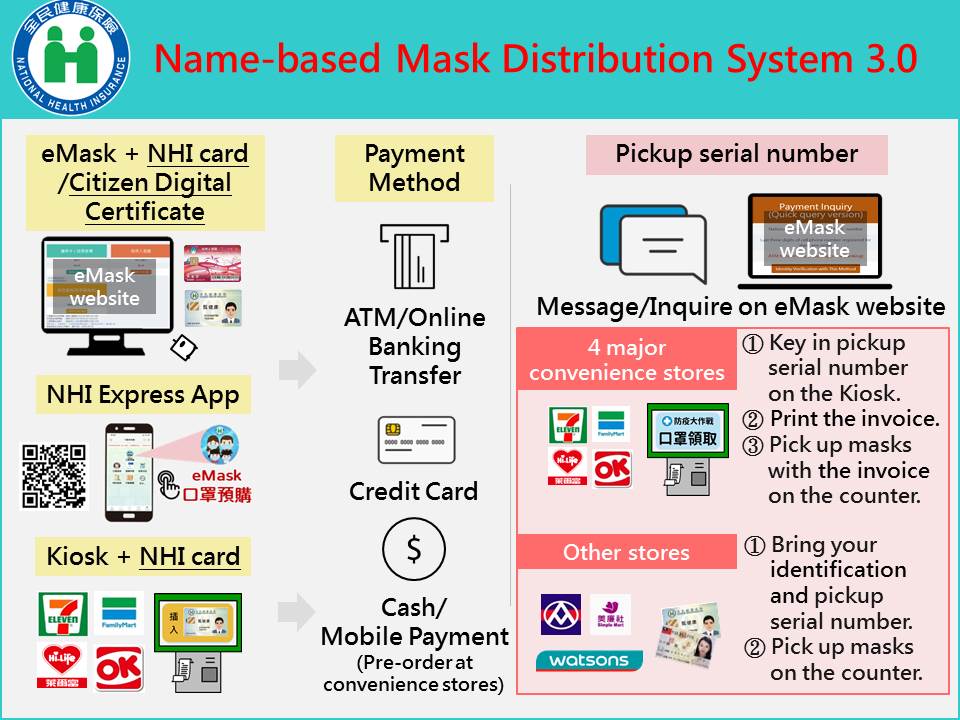
(Name-based Mask Distribution System 3.0)
(II) Face masks for official use:
- After the central government began requisitioning face masks at the end of January, the face masks were distributed to 22 local governments for use by epidemic prevention personnel in healthcare institutions, civil affairs units, firefighters, and police, ensuring the efficiency of supply and dispatch flexibility.
- Coordinated and matched suppliers of epidemic prevention supplies (such as forehead thermometers, face masks, isolation gowns, and medical alcohol) for use by healthcare institutions.
- A project was implemented on April 9, 2020 to provide every home care nurse with 2 N95 face masks and 1 isolation gown each week.
- Each nurse at general nursing homes received 1 N95 face mask and 1 isolation gown to replenish their inventory, so that they have the protection they need when they encounter a patient that is potentially infected with the novel coronavirus.

(Premier Su, Tseng-chang and Minister of Economic Affairs Shen, Jong-Chin inspected a new mask production line. Source: Executive Yuan)

(President Tsai, Ing-Wen inspected the logistics processes for mask. Source: Office of the President)
III. Increased the availability of epidemic prevention supplies through multiple channels:
(I) Provided guidance to special case manufacture or permit application of medical face mask, isolation gown, full-body protective clothing, forehead/ear thermometer, and COVID-19 test kit manufacturers, providing regulatory assistance and accelerating the review process.
(II) Announced reference documents on the application of special case manufacture for nucleic acid, antigen tests, and antibody tests, as well as ventilators for patients with respiratory failure or deteriorated respiratory function to address emergency use in the COVID-19 pandemic situation and increase domestic manufacturing capacity, so that the industry may use these documents as references.
(III) Launched the name-based rationing system for at-home COVID-19 test kits on April 28,2022. Under the rationing system, people can purchase test kits with their NHI card. In line with the restrictions on mask purchases imposed in the initial stage of the Name-based Mask Distribution System 1.0, the last digit of the ID number of an individual determined which days the individual can buy a rapid test kit. On June 13, restrictions regarding ID numbers ending in odd or even numbers under name-based rapid test distribution system were lifted.

(Isolation gown production line. Source: Office of the President)
IV. Monitor healthcare and testing capacity and reserve sufficient healthcare personnel:
(I) Monitor key healthcare resources daily, including: Special clinics for epidemic prevention, COVID-19 testing stations, number of ventilators available in case of an emergency, utilization of isolation rooms, and number of dedicated hospitals and beds.
(II) Extend the expiration date of licenses for medical practitioners and specialists, and suspend various evaluations for all healthcare personnel so that the focus can remain on epidemic prevention.
(III) Planned a four-stage preparedness strategy to expand capacity for patient treatment in response to epidemic developments.
- Treat patients in the negative-pressure isolation beds of isolation hospitals and response hospitals.
- Increase the number of designated wards.
- Begin using designated response hospitals for treatment.
- Plan centralized isolation centers.
(IV) Established COVID-19 patient hospitalization divergence/segregation and two-way referral principles, and established a two-way referral network for severe and mild cases.
(V) Encouraged all regional or higher level hospitals to establish P2+ laboratories, providing a local testing network to increase testing capacity and efficiency.
V. MOHW affiliated hospitals on the front line:
(I) Implement the quarantine measures for flights and ships arriving from regions/countries affected by COVID-19.
(II) Provide healthcare in quarantine facilities.
(III)As of April 30 ,2023, government quarantine facilities and local enhanced quarantine hotel were all released from requisition .

(Promoting the mask policy. Source: Executive Yuan)
- Created:2023-05-10
- Last Updated:2023-05-10
- Data Source:Hospital and Social Welfare Organizations Administration Commission
- Count Views: