Adequate testing capacity and precisely locate potentially infected individuals
Background
The COVID-19 pandemic is caused by a novel coronavirus of which the global community still has little understanding and studies are actively underway. However, due to the rapid spreading of the disease, over 3.7 million people worldwide have been diagnosed with COVID-19, and more than 260,000 people passed away from December 2019 to May 2020. As a large number of people present with the illness in such a short amount of time, many countries are experiencing medical staff work overload and the collapse of medical healthcare systems, leading to high mortality rate of confirmed cases in some countries. To limit the spread of COVID-19 and protect the medical healthcare system, to save more lives, Taiwan has handled the epidemic with great caution, and had smart testing policies in place prior to the occurrence of the outbreak. Not only so, but rolling plans and adjustments are also made towards the definition of suspected COVID-19 cases to ensure the testing criteria correlate with the most updated epidemiological investigation outcomes.

President Tsai Ing-wen inspects the laboratory in the Kunyang Office of the Taiwan Centers of Disease Control, Ministry of Health and Welfare.
Decisions and measures
I. Establishment of national testing network:
(I) In response to the future potential demands for large number testing works, the CECC has initiated stepwise upgrades of the overall testing capacity as well as refinements in the management and distribution processes of specimens.
(II) Currently, there are 263 designated testing facilities in Taiwan: North (113), Central (47), South (81), East (14) and offshore island (8). Taiwan now currently has a testing capacity of up to 236,113 specimens per day . The test kits Taiwan uses are molecular diagnostics with high sensitivity, which allows the result to be read after 4-6 hours. This method is widely adopted by other countries all over the world.
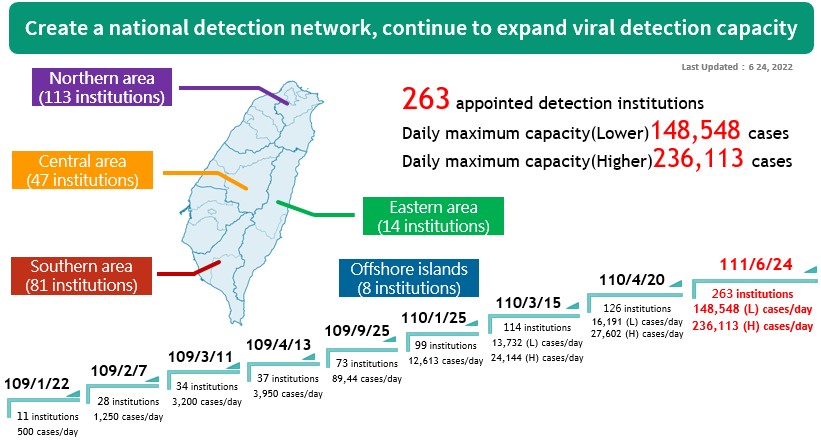
The national inspection network continues to expand its capacity.
II. Constructing community surveillance network and accurate testing strategies:
(I) Expanded testing: Encourage all regional hospitals or above within the nation to implement biosafety level 2 negative air pressure laboratories (BSL-2 negative air pressure laboratories) and BSL-2 (with BSC) laboratories that can provide a local testing network to expedite testing.
(II) Activating a plan for strengthening community surveillance: completion of community surveillance network and treatment by medical institutions at different levels according to the severity of the disease. The plan focuses on the reinforcing screening for high risk subjects. Dedicated wards or hospitals have also been designated for patients with mild or severe symptoms.
(III) Creating a map for community facilities (406 facilities) designated for specimen collection to provide easy access for the public to receive specialized evaluation and screening. Moreover, 53 hospitals have been designated for treatment of severe cases. Strengthening the community surveillance helps alleviate the burden on the hospitals.
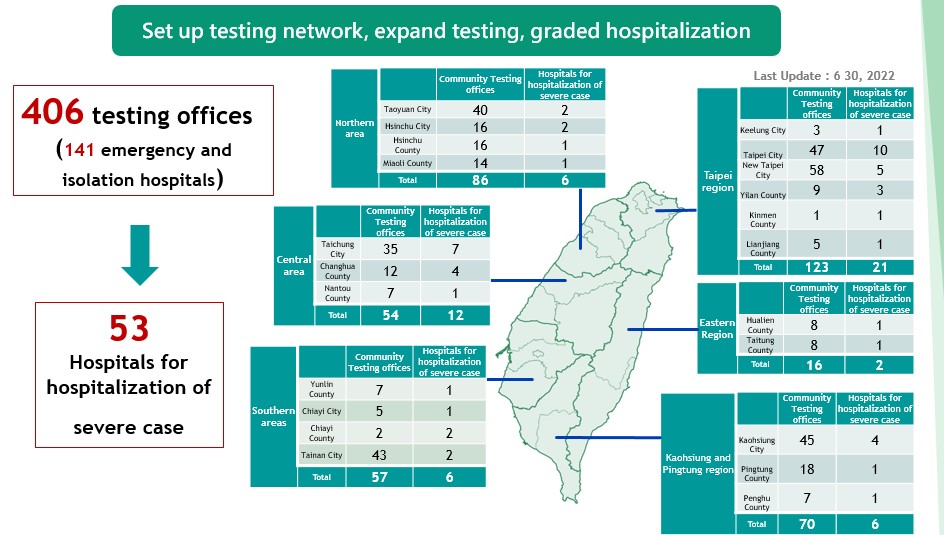
A community collection and inspection network continues to expand collection and inspection capacity.
(IV) Taiwan has carried out contact tracing in synch with the stepping up of international travel warnings are as follows:
1. Testing was extended to people entering Taiwan from Europe between March 3-14, with records for seeking medical attention to carry out virus screening and include them in home quarantine.
2. Testing extended to people entering Taiwan from the United States and Eastern Asia between March 8-18, with records for seeking medical attention to carry out virus screening and immediately include them into home quarantine.
(V) The CECC continually updates its “Recommendations for COVID-19 Case Definition, Specimen Collection, and Diagnostic Tests” to ensure the national testing criteria correlates with the most updated epidemiological investigation outcomes. Additionally, physicians are asked to report immediately cases that cannot be excluded for COVID-19 to carry out testing.
(VI) In response to the severity of the global COVID-19 outbreak and the risk of the Omicron variant spreading in Taiwan's communities, the CECC has continued to implement five enhanced surveillance measures since August 30, 2021, to detect domestic cases and break chains of transmission in a timely manner. These five measures are as follows:
1. Enhanced community surveillance;
2. Monitoring of specific high-risk workers at international airports/ports;
3. Waste water monitoring;
4. Surveillance on imported frozen food packaging at the border;
5. Investigations on prevalence of SARS-CoV-2 antibodies among blood donors.
(VII) In order to expedite testing for individuals under isolation or quarantine and to take relevant epidemic prevention measures in a timely manner, the CECC Expert Advisory Committee passed a resolution on April 12, 2022, to replace PCR testing with rapid testing for people in isolation and quarantine. According to the resolution, such individuals should take rapid tests and report the results themselves. Individuals testing negative on rapid tests would be released from isolation or quarantine when their isolation or quarantine period expires. In consideration of the age limit on who can use at-home rapid test kits, the CECC decided to give children under two years of age who are under isolation/quarantine a PCR test instead of a rapid test. People who have difficulty using at-home rapid test kits on their own will receive rapid tests arranged and performed by local governments.
III. Speeding up research and development of relevant test reagents and rapid test kits:
(I) As national and international experts and scholars have predicted the likelihood of COVID-19 progressing into an influenza-like infection, the CECC has set up an additional research and development team to tackle five major aspects accordingly: screening, vaccines, pharmaceuticals, prediction models and technical support platform to carry out research and development work division and integration. In addition, CECC collaborates with the national specimen database to offer usage upon application by national industries, research, and medical fields. Co-operation with the Biosafety level-3 laboratory (BSL-3) is also being done to contribute in the testing of virus, pseudo-specimens, specimens and viral plaque inhibitory tests. Apart from disease prevention research over the severe factors that influences the disease to help with finding the right treatment regime, more effective means in assisting the development of vaccine and quick testing kits are done through the analysis of antibody concentration evolution.
(II) The MOHW also plans to develop standardized formulas for screening and quick testing kits for companies to carry out efficacy evaluation of nucleic acid amplification products, in the hopes of propelling early phases of clinical trial progress and allowing the outcomes to be submitted for registration inspection as part of the documents for evaluation. Currently, there is already one nationally produced product (GeneReach nucleic acid test kit) that has completed clinical evaluation and being marketed. The test kit requires 85 minutes to achieve test results and has finished clinical trials, with both sensitivity and specificity over 95%, and is now ready for the next step in manufacturing.
Relevant links
- Created:2021-03-05
- Last Updated:2023-11-14
- Data Source:Centers for Disease Control, Ministry of Health and Welfare
- Count Views:
